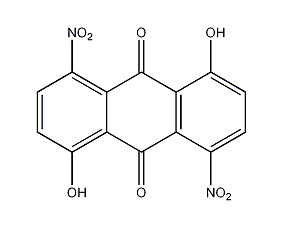
structural formula
| business number | 03lt |
|---|---|
| molecular formula | c14h6n2o8 |
| molecular weight | 330.21 |
| label |
1,5-dihydroxy-4,8-dinitroquinoneanthracene, 1,5-dihydroxy-4,8-dinitro-10-anthracenedione, 1,5-dihydroxy-4,8-dinitroanthracene-9,10-dione, 1,5-dihydroxy-4,8-dinitroanthrachinon, 4,8-dinitroanthrarufin, aromatic compounds |
numbering system
cas number:128-91-6
mdl number:mfcd00035827
einecs number:none
rtecs number:none
brn number:none
pubchem id:none
physical property data
none
toxicological data
none
ecological data
none
molecular structure data
1. molar refractive index: 75.52
2. molar volume (cm3/mol): 179.6
3. isotonic specific volume (90.2k ): 579.4
4. surface tension (dyne/cm): 108.2
5. polarizability (10-24cm3): 29.93
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): 2.9
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 8
4. number of rotatable chemical bonds: 0
5. number of tautomers: 10
6. topological molecule polar surface area 166
7. number of heavy atoms: 24
8. surface charge: 0
9. complexity: 545
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
has a pungent odor.
storage method
sealed iron drum packaging
synthesis method
it is produced by the interaction between 1,5-dichloroanthraquinone and phenol, followed by nitrification and hydrolysis. first add sodium hydroxide and phenol into the reaction kettle, and heat to 130~150°c to completely dissolve them. cool to 120°c, add 1,5-dichloroanthraquinone, react at 145~155°c to the end point, and obtain 1,5-diphenoxyanthraquinone through distillation, filtration, water washing and drying. then, add mixed acid and 1,5-diphenoxyanthraquinone into the nitrating kettle and perform nitration at 40°c to obtain 1,5-(2,4-dinitrophenoxy)-4,8-dinitroanthracene. quinone. add water and the nitrate obtained above to the hydrolysis kettle, stir evenly, adjust the volume, add 30% solution, raise the temperature to 95°c, keep it warm for 30 minutes, cool it below 50°c, filter, and wash with 3% sodium hydroxide solution until washed. no precipitation occurs after liquid acid precipitation. the filter cake is the finished product 1,5-dihydroxy-4,8-dinitroanthraquinone. the filtrate is subjected to acid precipitation and filtration to obtain 2,4-dinitrophenol.
purpose
dye intermediates. used to manufacture disperse blue 2bln dye, etc.

 微信扫一扫打赏
微信扫一扫打赏

